Introduction
Chemical etching is a process of selectively removing material from a metal or metal alloy surface by exposing it to a chemical solution. This process is commonly used in the manufacture of printed circuit boards, microelectronics, Nameplates, and other high-precision metal parts. The chemical solution reacts with the metal surface to dissolve or corrode specific areas, creating intricate patterns or designs.
Chemical etching is an economical, precise, and versatile alternative to other manufacturing techniques, such as stamping, laser cutting, or photolithography. The process can produce precise patterns with high resolution, minimal burrs, and no heat-affected zones, making it an attractive option for various applications. Furthermore, chemical etching can be performed on a wide range of metals, including stainless steel, brass, aluminum, and many others.
General Steps for Chemical etching
1. Cleaning
Cleaning is an important step in the chemical etching process, as it ensures that the metal surface is free of contaminants, oils, and other impurities that can interfere with the etching reaction. A clean surface is also essential for achieving a high-quality final product with sharp, defined edges and uniform etching depth.
The cleaning process typically involves the following steps:
1.1. Degreasing: Removes oils and other contaminants from the surface of the metal. Common degreasing agents include solvents, such as trichloroethylene or acetone, and aqueous solutions, such as alkaline cleaners.
1.2. Deoxidation: Removes oxidation and other forms of corrosion from the surface of the metal. This step is important for metals such as aluminum and stainless steel, which are prone to oxidation.
1.3. Rinsing: Removes any residue from the degreasing and deoxidation steps. This step is typically performed using distilled water to avoid introducing any additional contaminants.
1.4. Drying: Dries the metal surface to prevent any further oxidation or corrosion. This step is typically performed using hot air or a mechanical dryer.
The cleaning process may vary depending on the metal being etched and the specific requirements of the etching process.
Some common cleaning processes for various metals include:
- Aluminum: Aluminum surfaces can be cleaned using a degreasing solution, such as trichloroethylene or aqueous solutions, to remove any oils or other contaminants.
- Copper and Copper Alloys: Copper and copper alloys can be cleaned using a degreasing solution, such as trichloroethylene or aqueous solutions, followed by a cleaning solution, such as a nitric acid or aqueous solution, to remove any surface oxides.
- Steel: Steel surfaces can be cleaned using a degreasing solution, such as trichloroethylene or aqueous solutions, followed by a pickling solution, such as nitric acid or sulfuric acid solution, to remove any surface oxides.
- Stainless Steel: Stainless steel surfaces can be cleaned using a degreasing solution, such as trichloroethylene or aqueous solutions, followed by a cleaning solution, such as a nitric acid or aqueous solution, to remove any surface oxides.
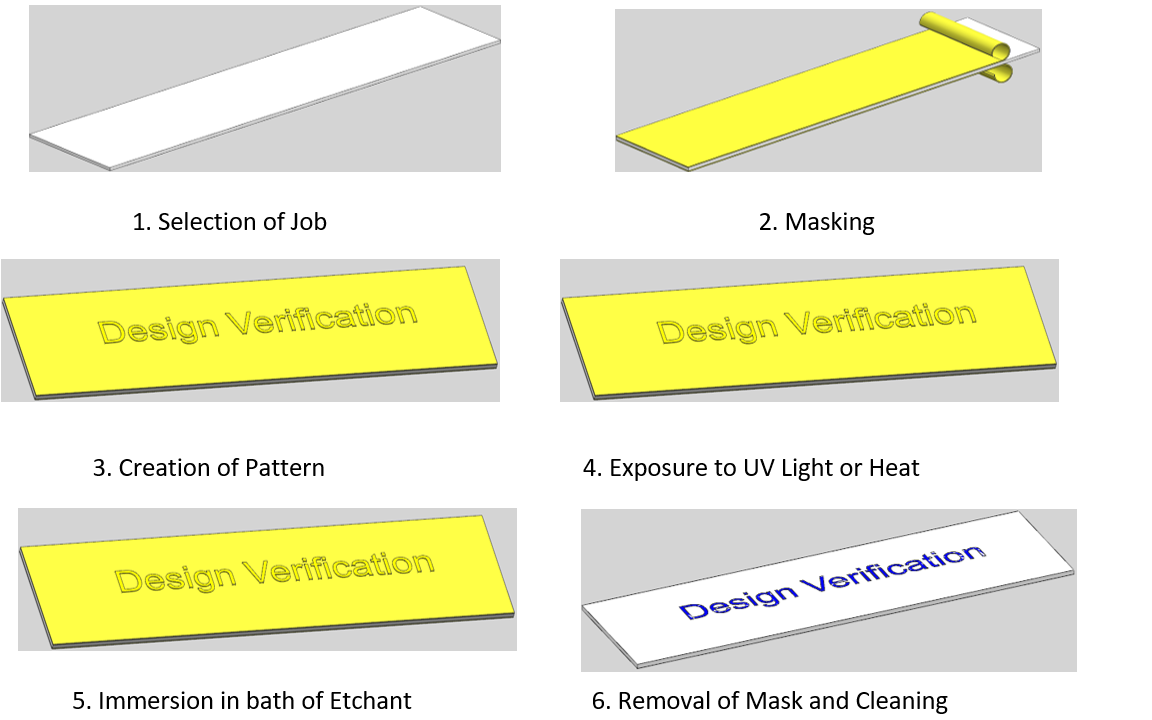
2. Masking
Masking is an important step in the chemical etching process that involves protecting certain areas of the metal surface from being etched. The purpose of masking is to create a specific pattern or design on the metal surface and to prevent the etching solution from reacting with areas that are not intended to be etched.
- Photoresist: Photoresist is a light-sensitive material that is used as a masking material in photolithography. It is commonly used in chemical etching processes that use acidic etching solutions, such as ferric chloride, and is suitable for use with metals such as stainless steel, copper, and aluminum. Photoresist is applied to the metal surface, exposed to light, and developed to create the desired pattern.
- Tape: Tape is a common masking material used in chemical etching. It is suitable for use with less aggressive etching solutions, such as ferric chloride, and is commonly used with metals such as stainless steel and aluminum. Tape is applied to the metal surface to create the desired pattern, and is easily removed after etching.
- Vinyl: Vinyl is another commonly used masking material in chemical etching. It is suitable for use with less aggressive etching solutions and is commonly used with metals such as stainless steel, aluminum, and brass. Vinyl is applied to the metal surface to create the desired pattern and is easily removed after etching.
- Rubber: Rubber is a flexible masking material that is used in chemical etching processes. It is suitable for use with a variety of etching solutions, including both acidic and alkaline solutions. Rubber is commonly used with metals such as stainless steel and aluminum, and is applied to the metal surface to create the desired pattern.
- Foil: Foil is a metal masking material that is used in chemical etching processes. It is suitable for use with a variety of etching solutions, including both acidic and alkaline solutions. Foil is commonly used with metals such as stainless steel and aluminum, and is applied to the metal surface to create the desired pattern.
The type of masking material used and the method of application will depend on the specific requirements of the etching process, including the complexity of the design, the etching solution used, and the metal that is etched.
3. Exposure
The exposure step in the etching process is where the masking material is exposed to light or heat in order to activate it. The exposed areas will become more resistant to etching, while the unexposed areas will be removed during the subsequent etching step. The pattern exposure step is crucial in determining the final shape and design of the etched product.
The following are the general steps involved in exposure:
- Prepare the photoresist: The photoresist must be applied to the metal surface, as described in the previous answer. The photoresist must be allowed to dry for the specified period of time.
- Create the pattern: The pattern can be created in several ways, including photolithography, screen printing, or hand drawing. The method used will depend on the desired final product, the equipment available, and the complexity of the pattern.
- Photolithography: In photolithography, a mask or stencil is placed over the photoresist and the metal surface is exposed to light. The light passes through the mask or stencil, exposing the photoresist to the areas where light is able to penetrate.
- Screen printing: In screen printing, a stencil is used to apply ink to the photoresist, exposing the photoresist to the areas where the ink is applied.
- Hand drawing: In hand drawing, a pen or marker is used to draw directly on the photoresist, exposing the photoresist to the areas where the pen or marker is applied.
- Expose the photoresist: Once the pattern has been created, the photoresist must be exposed to light or heat. The exposure time will depend on the type of photoresist being used, the exposure source, and the desired depth of the etching.
- Develop the photoresist: After exposure, the photoresist must be developed to remove the unexposed areas. This can be done by washing the photoresist with a solvent or by using a developer solution.
4. Etching
it involves immersion of the masked metal in a bath of etching solution, which reacts with the unprotected areas of the metal surface to dissolve or corrode the metal. This reaction continues until the desired pattern or design is achieved.
The etching solution used in the process depends on the metal being etched and the desired outcome and can contain a variety of chemicals.
The chemicals used in the etching process can vary depending on the metal being etched and the desired outcome. Some common chemicals used in chemical etching include:
- Acids: Hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), and phosphoric acid (H3PO4) are commonly used in etching processes. The type of acid and the concentration used will depend on the metal being etched and the desired etching rate.
- Bases: Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are commonly used in etching processes for metals such as aluminum and nickel.
- Salts: Salts such as ferric chloride (FeCl3) and cupric chloride (CuCl2) are used in etching processes for copper and other copper-based metals.
- Other chemicals: Other chemicals, such as hydrogen peroxide (H2O2) and ammonium persulfate (NH4)2S2O8, can be used in etching processes to improve the etching rate or to modify the etching solution.
Factors that control the Etching process:
- Type of metal: The type of metal being etched is a key factor that affects the etching rate and the final pattern. Different metals have different etching characteristics and require different etching solutions and conditions.
- Type of etching solution: The type of etching solution used is also a key factor that affects the etching rate and the final pattern. Different etching solutions have different etching rates and characteristics and are used for different metals and desired outcomes.
- The concentration of the etching solution: The concentration of the etching solution can affect the etching rate and the final pattern. A higher concentration of the etching solution will generally result in a faster etching rate, while a lower concentration will result in a slower etching rate.
- Temperature: The temperature of the etching solution can also affect the etching rate and the final pattern. Higher temperatures can result in a faster etching rate, while lower temperatures can result in a slower etching rate.
- Immersion time: The immersion time is the amount of time the metal is immersed in the etching solution, and it affects the depth of the etch. A longer immersion time will result in a deeper etch, while a shorter immersion time will result in a shallower etch.
The following are some general guidelines for etching times with specific chemicals to get the specific depth of etching:
Ferric Chloride: Etching times with ferric chloride can range from a few minutes to several hours, depending on the metal being etched and the desired depth. For copper, etching times can range from 10-30 minutes for a depth of 0.001-0.005 inches.
Nitric Acid: Etching times with nitric acid can range from a few minutes to several hours, depending on the metal being etched and the desired depth. For stainless steel, etching times can range from 10-30 minutes for a depth of 0.001-0.005 inches.
Hydrogen Peroxide: Etching times with hydrogen peroxide can range from a few minutes to several hours, depending on the metal being etched and the desired depth. For aluminum, etching times can range from 30 minutes to several hours for a depth of 0.001-0.005 inches.
Ammonium Persulfate: Etching times with ammonium persulfate can range from a few minutes to several hours, depending on the metal being etched and the desired depth. For aluminum, etching times can range from 30 minutes to several hours for a depth of 0.001-0.005 inches.
Sodium Hydroxide: Etching times with sodium hydroxide can range from a few minutes to several hours, depending on the metal being etched and the desired depth. For aluminum, etching times can range from 30 minutes to several hours for a depth of 0.001-0.005 inches. - Agitation: Agitation of the etching solution can help to improve the etching rate and uniformity. The type and intensity of agitation will depend on the etching solution and the desired outcome.
- Masking material: The type of masking material used can affect the final pattern and the ease of the mask removal process. Different masking materials have different etching characteristics and require different removal methods.
5. Removing the Mask
After the etching process is complete, the next step is the removal of the masking material. This is an important step in the chemical etching process as the masking material protects specific areas of the metal surface from being etched, and its removal reveals the final pattern or design on the metal surface.
There are several methods for removing the masking material, including mechanical removal, solvent removal, and thermal removal. The method used will depend on the type of masking material used, the metal being etched, and the desired final surface condition of the metal.
Mechanical removal involves using tools such as scrapers or brushes to physically remove the masking material from the metal surface. Solvent removal involves immersing the metal in a solvent that dissolves the masking material, allowing it to be easily removed. Thermal removal involves heating the metal to a temperature that causes the masking material to evaporate or break down, leaving the metal surface clear.
6. Rinsing and Drying
After the masking material is removed, the next step in the chemical etching process is typically rinsing and drying the metal. This step is important as it removes any residual etching solution or masking material from the metal surface and prepares it for any post-treatment processes.
The metal is typically rinsed with water or another cleaning solution to remove any residual etching solution or masking material. The rinse solution and rinse conditions, such as the temperature, flow rate, and duration, are carefully controlled to ensure that the metal is thoroughly cleaned.
After rinsing, the metal is typically dried using a process such as air-drying, spin-drying, or hot-air drying. The drying process is important as it removes any remaining moisture from the metal surface, which can affect the final surface condition of the metal and the performance of any subsequent post-treatment processes.
Positive points of the Chemical Etching process
- High precision: Chemical etching allows for precise control over the etching process, resulting in precise and accurate patterns and shapes.
- Repeatability: Chemical etching is a highly repeatable process, allowing for the consistent production of high-quality parts.
- Cost-effectiveness: Chemical etching is a cost-effective method for producing parts with complex shapes, especially compared to traditional machining methods.
- Versatility: Chemical etching can be used on a wide range of metals, including stainless steel, copper, aluminum, brass, nickel, gold, and silver.
- No heat-affected zone: Chemical etching does not produce a heat-affected zone, making it suitable for delicate and heat-sensitive materials.
- No mechanical stress: Chemical etching does not produce mechanical stress on the etched material, making it ideal for thin and brittle materials.
- Complex shapes: Chemical etching can produce complex and intricate shapes, making it suitable for a wide range of applications, including electronics, medical devices, and aerospace components.
Negative points of the Chemical Etching process
Chemical etching is a highly versatile and cost-effective process, but there are also some negative points to consider, including:
- Limited thickness: Chemical etching is limited to thin metal sheets and can only etch to a certain depth, typically in the range of 5-200 micrometers.
- Complex process: Chemical etching can be a complex process, requiring careful control over the etching conditions and a thorough understanding of the materials and etching solutions used.
- Hazardous chemicals: The etching solutions used in chemical etching can be hazardous and require careful handling and disposal.
- Equipment cost: The equipment required for chemical etching can be expensive, making it cost-prohibitive for small-scale or low-volume production.
- Limited material selection: Chemical etching is limited to specific metal materials and may not be suitable for other types of materials, such as plastics or ceramics.
- Surface finish: Chemical etching can result in a rough or porous surface finish that may require additional processing to achieve the desired surface finish.
- Time-consuming: Chemical etching can be a time-consuming process, especially for complex shapes or deep etches.
- Limited scalability: Chemical etching is limited in scalability and may not be suitable for high-volume production, as it requires frequent mask changes and maintenance of the etching equipment.